Saccharin
Discovery
:
Saccharin is firstly discovered by russian chemist constantin fahlberg (22 dec. 1850 -15 aug. 1910 ) in 1789.
They have accidently discovered saccharin .
When constantin fahlberg had worked a long time on the compounds products of coal-tar. One evening he was worked in laboratory till late ,then he was went for dinner without hand wash , he was surprised when they broke a piece of bread and put into mouth , it is undoubtedly sweet in taste .
They was surprised but not exactly understand why it is happening . they went for wash mouth and dried with napkin after wash , surprisingly the napkin is also become sweet, then they put fingers into mouth cause of fingers had touched before their work in laboratory , surprisingly fingers are sweet in taste ,
Then he was understand something but not clearly , immediately they dropped dinner and went in laboratory they check the taste of all solutions they had worked on.
One of them solutions they found sweet taste ,after that they worked on that particular saccharin solution and make best way of synthesis for saccharin scientifically and commercially.
After that their work for many months results into discovery of saccharin .
Generally the sucrose , glucose ,
fructose type natural saccharides are used for sweet test , but the saccharin
is a artificial sweetening
agent.
Sweetening
agent :
Definition : synthetic organic compound which have a sweet test called sweetening agent .
Or
Artificial Non-saccharide compound which have a sweet test , known as sweetening agent.
Or
Some organic synthetic compounds are not saccharides but they have sweet test, these are called sweetening agents.
|
Natural sweetening agent Some natural organic compounds have naturally sweet test, contains calories and nutrients these are known as natural sweetening agent . Eg. Glucose , sucrose , fructose , honey etc. |
Artificial sweetening agent Some artificial organic compound (but not saccharide) have a sweet test , not have any nutrient value , known as artificial sweetening agents. Eg. Sachharin , aspartame, sucrolose , alitame , p-anisyl-urea , dulsin etc. |
Saccharin is a non-nutritive sweetening agent
It is a crystalline solid compound .
Saccharine is 500 times sweeter than our regular sugar.
Practically it is insoluble in water but , sodium salt of saccharin is soluble in water .
Dilute solution of saccharine is sweet in taste but , highly concentrate (0.8%) solution of saccharin is bitter in taste.
Saccharine is synthesized from toluene and anthranilic acid .
Chemical
properties :
IUPAC name : 1,1-dioxo-1,2-benzothiozol-3-one.
CAS number ; 81-07-2.
Structure formula :
Physical
properties :
Molecular formula : C7H5NO3S.
Molecular weight : 183.18 gm/mole.
Melting point : 228.8 0C .
Boiling point : 226-229 0C .
Solubility : sodium salt of saccharin soluble in water ( 100 gm/liter at 200C )
Slightly soluble in chloroform and benzene.
Also soluble in ethanol and acetone .
Color : colorless or white .
State : crystalline solid.
Odor : sweet aromatic odor or odorless.
Synthesis of saccharin
Two
methods for synthesis of saccharine is given bellow,
Method-1
: synthesis of saccharin from toluene .
Statement
:
the condensation reaction of toluene with chloro sulphonic acid at 0-5 0c temperature gives two isomers ortho and pera- toluene-sulphonyl-chloride. Then reaction of ortho isomer with ammonia forms o-toluene-sulphonamide , oxidation of o-toluene-sulphonamide in the presence of alkaline KMnO4 gives o-amino-sulpho-benzoic acid. Heating of o-amino-sulpho-benzoic acid yields saccharin.
Chemical
reaction :
Method-2
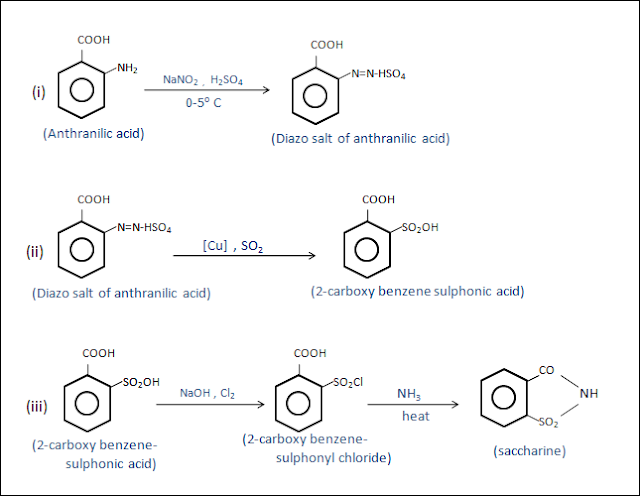
: synthesis of saccharin from anthranilic acid .
Statement
:
Diazotization of anthranilic acid with NaNo2 at 0-5 0c , in the presence of H2So4 gives diazo-salt. Then Diazo-salt reacted with liquid SO2 , in the presence of copper catalyst forms 2-carboxy-benzene-sulphonic acid . after that reaction of 2-carboxy-benzene-sulphonic acid with Cl2 and NaOH gives 2-carboxy-benzene-sulphonyl chloride, then reaction of 2-carboxy-benzene-sulphonyl chloride with ammonia yields saccharin.
Chemical
reaction :
(1) Saccharine is used by diabetic and fat person in the place of sugar , because of no nutrient value of saccharin.
Saccharin is also used in following given bellow ,
(2) Jams
(3) In Production of jellies.
(4) Cookies .
(5) Candies .
(6) Medicines.
(7) Toothpaste.
(8) Cheap cold drinks.
(9) Tinned fruit.







![ATENOLOL [TENORMIN] - medicinal drug -study everything.](https://blogger.googleusercontent.com/img/b/R29vZ2xl/AVvXsEgGTsBGKNaP_tE51nfYj2h3fKdZpO02gO5BeGfItb45xXsb4Gs_pu7ojGb8kc5WTo3e8qZHTxUwGLRwVrpBxzY-Bld1uR2rsmHRYEj3fX0uV4dEYiMa_46l17eSkXpgPnWb3pgGvDGvkqBU/w100/structural+formula+atenolol.PNG)

0 Comments
please do not enter any spam link in the comment box.