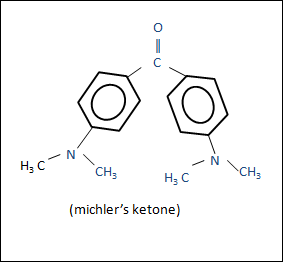
Michler’s ketone
Michler’s ketone is given by
Wilhelm michler ( December 1846 – November 1889). German chemist .
Nationality : german
michler’s ketone
Michler’s ketone is an organic compound which widely used as intermediate in the synthesis of dyes.
Chemical properties :
IUPAC NAME : bis [4-(dimethyl amino ) phenyl ] methanone.
CAS NUMBER : 90-94-8
STRUCTURE FORMULA :
PHYSICAL PROPERTIES :
Molecular formula : C17H20N2O.
Molecular weight : 268.16 gm/mole .
Melting point : melting point is between 173 0c – 179 0c .
Boiling point : 360 0c .
Solubility : insoluble in water or partially soluble.
Soluble in alcohol and pyrimidine , partially soluble in ether .
Color : colorless , some what bluish or bluishgreen color .
State : crystalline solid .
Odor : not specific odor or odorless.
Synthesis of michler’s ketone
Statement : the reaction of dimethyl aniline(C6H5N Me2 ) with phosgene(COCl2) or triphosgene , yield’s ketonic compound which is firstly discovered by or synthesized by Wilhelm michler ; the ketone is known as michler’s ketone .
Chemical reaction :
Uses of michler’s ketone
Michler’s ketone is widely used in synthesis of dyes as a intermediate .
Their reaction with aniline gives many dyes , eg. Methyl violet.
The absorption properties of michler’s ketone is good that is way it’s used in the photoreactions.
Condensation reaction of michler’s ketone with many compounds gives different dye or color.
Note
: disbandment of michler’s ketone harmful for living organisms specially
animals .
It is not directly effect on body but become
decomposition of michler’s ketone into CO and Co2 it is harmful for our
body .
[અસ્તુ:]





![auramine-o dye or basic yellow-2 dye or diarylmethane dye or bis [4-(dimethyl amine ) phenyl ] – methanimium chloride. -synthesis - study everything](https://blogger.googleusercontent.com/img/b/R29vZ2xl/AVvXsEiUuUnnqOn6NnVrilGzE4o8flSLV681i_5-_72hcw9qHsj9ywHb2JdaJmNpo_rfJwH7V-QYSbI9rSvNqX-GqY41rD0bG7bbjRGramR3ffSIbZWp1pggwr-P-KTY91ZDh3i79x8wS9tsckUQ/w100/structure+formula.PNG)
![ATENOLOL [TENORMIN] - medicinal drug -study everything.](https://blogger.googleusercontent.com/img/b/R29vZ2xl/AVvXsEgGTsBGKNaP_tE51nfYj2h3fKdZpO02gO5BeGfItb45xXsb4Gs_pu7ojGb8kc5WTo3e8qZHTxUwGLRwVrpBxzY-Bld1uR2rsmHRYEj3fX0uV4dEYiMa_46l17eSkXpgPnWb3pgGvDGvkqBU/w100/structural+formula+atenolol.PNG)

0 Comments
please do not enter any spam link in the comment box.