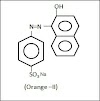
ORANGE-II [β-NAPHTHOL ORANGE OR 2-NAPHTHOL ORANGE]
WHAT IS DYES ? [5.1]
Definition : generally dye is a colored organic compound or
mixture which used for imparting color to the substrate or objects like paper,
plastic , cloths , and leather etc.
The long time stability is required for good
quality of dye.
The dyed substrate should be
resistant to a normal cleaning procedures and stable to light.
ORANGE-II [β-NAPHTHOL ORANGE OR 2-NAPHTHOL ORANGE]
Orange-II dye is a mono-azo (-N=N-) acidic (here the acid is SO3H) dye.
It is synthesized from sulphanilic acid.
CHEMICAL PROPORTIES :[ 5.2]
IUPAC NAME : 4-( 2-hydroxy-1-naphthylazo )benzene
sodium sulphonate.
PHYSICAL PROPERTIES :[5.3]
MOLECULAR FORMULA : C16H11O4N2Na S
MOLECULAR WEIGHT : 350.32
gm/mole.
MALTING POINT : 164OC.
BOILING POINT : sorry for
inconvenience we will give update as possible as soon.
SOLUBILITY : very soluble in
water ( 116 gm/l at 30oc ).
Slightly soluble in ethanol and
acid(hcl).
COLOR : orange.
STATE : crystalline powder.
ODOR : odorless.
USES OF ORANGE-II DYE : [5.5]
SYNTHESIS OF ORANGE-II [5.4]
Synthesis of orange-ii from
sulphanilic acid.
STATEMENT : in
the reaction first the sulphanilic acid dissolve in HCL & diazotized with
NaNO2 at 0-5OC temperature, after that these reactant
react with β-naphthol at that time the temperature of entire system is 8oc,
these reaction will take a two hours , then the temperature raised from 8 to 16oc
. at that time the pH of solution is about 9.1 to 9.4.
PH IS lowered by
adding acidifying substance (HCL) , yields orange-ii dye , further filtration
and the drying process gives main product ( pure ) orange-ii dye.
USES OF ORANGE-II DYE : [5.5]
Orange-ii dye is used widely for
dying many numbers of substrate given below.
(i)
Cotton
(ii)
Wool
(iii)
Fibres
(iv)
Silk
(v)
Paper
(vi)
Leather
NOTE :
some dyes are ( azo dyes ) toxic in
nature it is require to take care when use these type of dyes.
GENERAL
INFORMATION [5.6]
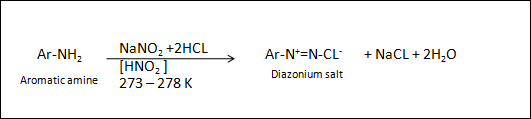
DIAZOTIZATION
REACTION :
STATEMENT : diazotization is the reaction in which
the primary aromatic amine converts into diazonium(N=N) salt.
In other words
The reaction of primary aromatic
amine with NaNO2 and HCL yields diazotized product.
Diazonium salt is shown by
general formula as Ar-N2+x-
Where x = CL-, Br -,
HSO4- , BF4- , etc. Group is called
diazonium group.
In their nomenclature the suffix
diazonium is applied to main hydrocarbon and then the name of negative ion is
written.
Primary aliphatic amine forms
highly unstable alkyl diazonium salt,
While primary aromatic amine
forms arene diazonium salt it is very stable compare to the aliphatic alkyl
diazonium salt cause of resonance.
Example : diazotization of aniline.
STATEMENT : the reaction of aniline with NaNO2
and HCl, HNO2 is used
as a catalyst , 273 – 278 k temperature yields benzene diazonium chloride (salt).
Benzene diazonium salt is very
unstable at higher temperature , so it is necessary to keep the temperature at
273 – 278 k.
see also :
what is science ? all about science. [click here].
ATENOLOL [TENORMIN] - medicinal drug [click here].
ADRENALINE [EPINEPHRINE] - medicinal drug -hormone [click here].
crsodine - G or (2,4-diamino-azobenzene) or mono-azo- basic dye [click here].
auramine-o or yellow dye or diarylmethane dye or bis [4-(dimethyl amine ) phenyl ] – methanimium chloride. [click here]
[અસ્તુ:]







![auramine-o dye or basic yellow-2 dye or diarylmethane dye or bis [4-(dimethyl amine ) phenyl ] – methanimium chloride. -synthesis - study everything](https://blogger.googleusercontent.com/img/b/R29vZ2xl/AVvXsEiUuUnnqOn6NnVrilGzE4o8flSLV681i_5-_72hcw9qHsj9ywHb2JdaJmNpo_rfJwH7V-QYSbI9rSvNqX-GqY41rD0bG7bbjRGramR3ffSIbZWp1pggwr-P-KTY91ZDh3i79x8wS9tsckUQ/w100/structure+formula.PNG)
![ATENOLOL [TENORMIN] - medicinal drug -study everything.](https://blogger.googleusercontent.com/img/b/R29vZ2xl/AVvXsEgGTsBGKNaP_tE51nfYj2h3fKdZpO02gO5BeGfItb45xXsb4Gs_pu7ojGb8kc5WTo3e8qZHTxUwGLRwVrpBxzY-Bld1uR2rsmHRYEj3fX0uV4dEYiMa_46l17eSkXpgPnWb3pgGvDGvkqBU/w100/structural+formula+atenolol.PNG)

3 Comments
Very useful information of your articles thank you 👌👌👌
ReplyDeletethanks bro. for appreciate me
DeleteYtuemoOplisko Steven Brosee https://wakelet.com/wake/4ycDOZhhClupE3SioRX-F
ReplyDeletewacompkonsti
please do not enter any spam link in the comment box.