ELECTROCHEMISTRY
[PART-1]
contents :-
electrochemistry definition-
general information-
metathetical reaction-
redox reaction –
classical concept of redox reaction –
electronic concept of redox reaction –
physical observation of electrochemical
cell –
here the topic is electrochemistry but firstly we have to know about basics of the particular topic . so firstly we discus about type of reactions and redox reaction. Because of there is a lots of concepts of electrochemistry based on redox reaction.
Definition : “electrochemistry is the study of production of
electricity “
The electricity is released during spontaneous chemical reaction.
The produced electrical energy is used for bring about non-spontaneous chemical transformations.
The widely uses of electrochemical methods into the production of many metals like sodium hydroxide , fluorine , chlorine etc.
We can see in our regular activity we use the batteries and fuel cells this is what ?
This is because of conversion of chemical energy into electric energy . the cells and batteries are used as large scale in various devices and tools .
Eg. Mobile phone , tv remotes , earphones , digital camera , laptops , trimmer etc.
As we know we all connected with that type of things so we can easily understand .
Types of chemical reactions :
What is chemical reactions :
Chemical
reaction is The reaction in which two or more reactant or substances are react
with each other and gives product called chemical reaction.
Or in other words
The scientific study of reaction in which
two or more reactant or substances react with each other and
gives product .
Here we discus about two type of chemical reactions , first one is the metathetical reaction and second is redox reaction.
Metathetical reaction :
That type of reaction in which no changes occur in oxidation number but become changes in partner, called metathetical reaction.
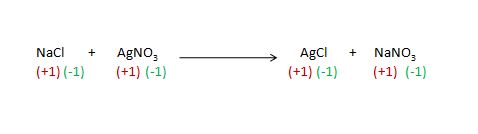
for easily understand look at the given chemical reaction in which there I given the oxidation number in below the reactants and particular molecule.
There is a no change occur in oxidation number but as we can see the changes in partner .
Detail explanation on changing or no-changing oxidation
number in given chemical reaction and changing in partner.
other way in reactant NaCl the oxidation number of Cl is (-1) and oxidation number of Cl in product(AgCl) is a also (-1) .
same case apply to the other reactant AgNO3
in the reactant AgNO3 the oxidation number of Ag is (+1) and also the oxidation number of Ag in product (AgCl) is (+1) .
other way the oxidation number of NO3 in reactant is (-1) and also in product (NaNO3) the oxidation number of NO3 is (-1) .
detail explanation of changing in partner (during chemical reaction
process) :
Other way in the reactant AgNO3 partner of Ag is NO3 but in product there will be replaced by the cl on the place of NO3 .
Redox reaction :
Classical concept of redox reaction :
Oxidation :
That type of reaction in which during process adding of oxygen(o) and removal of hydrogen[H] is called oxidation.
Reduction :
That type of reaction in which during process adding of hydrogen[H] and removal of oxygen(o) is called reduction.
Electronic concept or transfer of electron ( redox reaction ) :
Oxidation :
1) The
reaction in which removal of electrons or electron released is called oxidation
.
Eg.
In above reaction we can see the removal of electron.
Or in other words
2) that type of reaction in which oxidation number of an element is increases also called oxidation.
Eg.
In above reaction we can see the increases in oxidation number . the oxidation number of Na is increases from 0 to +1 .
Reduction :
1 ) The reaction in which electrons are gained is called
reduction.
Eg.
In above reaction we can see the electron has gained .
Or in other words
2 ) that type of reaction in which the oxidation number of an element is decreases is called reduction.
physical
observation of electrochemical cell :
requirement : zn metal strip , two beakers ,aqueous solution of CuSO4 .
fill the two beakers with the aqueous solution of CuSO4 .
then put the Zn strip into beaker like shown in figure .
-The intensity of the color of the CuSO4 solution decreases.
-the solution is become hot or solution is heated .
-corrosion of zn strip
-cu metal is found at the bottom of beaker.
If you have any query you can also leave comment I will give you solution for you query.
“Thank you”
[અસ્તુ:]












![auramine-o dye or basic yellow-2 dye or diarylmethane dye or bis [4-(dimethyl amine ) phenyl ] – methanimium chloride. -synthesis - study everything](https://blogger.googleusercontent.com/img/b/R29vZ2xl/AVvXsEiUuUnnqOn6NnVrilGzE4o8flSLV681i_5-_72hcw9qHsj9ywHb2JdaJmNpo_rfJwH7V-QYSbI9rSvNqX-GqY41rD0bG7bbjRGramR3ffSIbZWp1pggwr-P-KTY91ZDh3i79x8wS9tsckUQ/w100/structure+formula.PNG)
![ATENOLOL [TENORMIN] - medicinal drug -study everything.](https://blogger.googleusercontent.com/img/b/R29vZ2xl/AVvXsEgGTsBGKNaP_tE51nfYj2h3fKdZpO02gO5BeGfItb45xXsb4Gs_pu7ojGb8kc5WTo3e8qZHTxUwGLRwVrpBxzY-Bld1uR2rsmHRYEj3fX0uV4dEYiMa_46l17eSkXpgPnWb3pgGvDGvkqBU/w100/structural+formula+atenolol.PNG)

0 Comments
please do not enter any spam link in the comment box.